【Update】Results of the First IDA (Ivermectin, Diethylcarbamazine, and Albendazole) Impact Survey for the Lymphatic Filariasis Campaign in East New Britain Province
Results of the First IDA Impact Survey
In East New Britain Province (ENBP), the first round of Mass Drug Administration (MDA) was carried out in December 2019, followed by the second round in June 2022, both exceeding the WHO-recommended coverage rate of 65.0%. After two rounds of MDA, the first IDA Impact Survey (IIS)*1 was conducted in late April last year and successfully completed in June to evaluate the effectiveness of these MDA rounds. Previous articles have covered the full-day training session*2 and Training of Trainers and the survey launch. (See the link below.)
The survey covered 3,160 individuals from 30 randomly selected villages across the province. Participants were tested for Lymphatic Filariasis (LF) antigens using the Filariasis Test Strip (FTS). Those who tested positive underwent additional nighttime blood sampling to microscopically detect microfilariae (Mf)*3 in their bloodstream, which can be transmitted to mosquitoes during blood feeding. The survey results revealed that 73 out of 3,160 individuals (2.3%) tested positive for FTS. Among them, additional nighttime blood sampling detected Mf in only two individuals, both from the same area in Pomio District. Based on these findings, WHO deemed the survey a success ("Pass"), confirming that IDA-based MDA has effectively curbed LF transmission in ENBP. However, since the Mf positivity rate in the affected area exceeded 1% (2/105 cases), WHO recommended an additional targeted MDA for this region.
Impact of Joint Efforts
The IDA Impact Survey, which incorporated both FTS testing and nighttime blood sampling for Mf detection, was the first of its kind in Papua New Guinea. Its success was largely due to the strong leadership of the Provincial Health Authority (PHA) and the close collaboration among all involved organisations.
The IDA impact study is a localised investigation. While it is smaller in scale compared to the MDA implementation, it faces similar challenges, such as geographical issues and budget constraints. In particular, for FTS-positive individuals requiring nighttime blood sampling after 9 PM, several concerns arose—securing informed consent, ensuring the timely transport of blood samples, preparing specimens efficiently, managing time constraints, and addressing safety issues. Despite these challenges, PHA, as the leading organisation, effectively coordinated efforts with car hire companies and the Papua New Guinea Institute of Medical Research (PNG-IMR) to ensure the smooth execution of the survey. As a result, the survey was completed as planned, and all collected blood samples underwent Mf testing by PNG-IMR laboratory technicians.
Additionally, in the remote Pomio District—where the survey coincided with the early rainy season, making conditions even more challenging—the PHA team in Kokopo conducted pre-survey training for local health workers. Following this preparation, the survey was successfully carried out over an 11-day period. This achievement was made possible entirely due to the strong leadership and cohesive teamwork of the PHA.
The JICA-LF Team played a crucial role in supporting the survey by providing essential materials, arranging car hire and small boats, funding survey operations, and conducting pre-survey training. Additionally, the team facilitated preliminary data explanations and verification. All FTS kits and the three-drug IDA regimen were supplied through WHO.
Future Plans and Next Steps
Future activities in ENBP will prioritise the focal MDA in areas requiring further intervention based on the survey findings. Also, preparations for the second IDA Impact Survey, scheduled for 2026 and beyond, will incorporate lessons learned and identified improvements to ensure its smooth execution.
To achieve LF elimination, three IDA Impact Surveys must be conducted biennially following the completion of two rounds of IDA-based MDA, with all surveys meeting the required "Pass" criteria. Furthermore, in areas where Mf-positive cases were detected, additional localised MDA may be necessary. The LF Team will continue working closely with all stakeholders to systematically advance these efforts and move toward the successful elimination of LF in the target regions.
*1 The term "IDA" is derived from the initials of the three drugs— Ivermectin, Diethylcarbamazine (DEC), and Albendazole. The evaluation survey conducted after MDA using these three drugs is referred to as the "IDA Impact Survey (IIS)."
*2 Two health workers who were absent from the training session received follow-up training from their colleagues. Also, in the remote Pomio District, a separate pre-survey training session was conducted by the PHA team for seven health workers.
*3 Since microfilariae (Mf) exhibit nocturnal periodicity, nighttime blood sampling is required for their detection.
Timeline
| January 2024 | Finalisation of the Survey Implementation Plan (Micro Plan). |
| February-April 2024 | Procurement and sorted procured items. |
| End of April 2024 | Training for health workers. |
| End of April-May 2024 | IIS activity 1 (Approx. 10 days, excluding some areas in Pomio District. Microscopic Examination for Mf Detection in Collected Blood Samples. |
| June 2024 | IIS activity 2 (Approx. 11 days in some areas in Pomio District). |
| June-July 2024 | Microscopic Examination for Mf detection in Samples from the IIS activity 2. Data completion |
| July 2024 | Review and Feedback on Survey Report from WHO. |
For more information

Health workers conducting nighttime blood sampling near Kokopo

PNG-IMR laboratory technicians examining samples for Mf detection
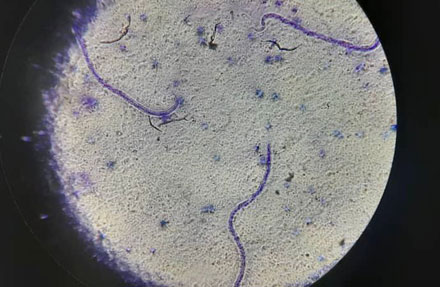
Confirmed Mf cases identified in the survey

PHA team conducting the survey in the remote Pomio District
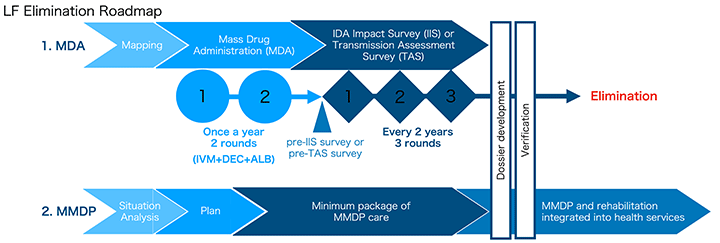
Roadmap for LF elimination.