- Home
- Technical Cooperation Projects
- Index of Countries
- Asia
- Indonesia
- Project for Enhancing Drug and Food Safety
- Project News
- JICA Project Takes Part in PV Basic Module Workshop
Project News
2022-03-11
JICA Project Takes Part in PV Basic Module Workshop
JICA Project and the Indonesian FDA conducted the Pharmacovigilance (PV) Basic Module Workshop as the first series of PV Graded Modules, with the aim of improving PV competence to strengthen drug safety. In PV workshops 2020, several requests are raised from the participants to prepare level-graded PV learning materials to deepen their understanding. The basic level modules are expected to guide pharmaceutical companies, BPOM officers, especially healthcare professionals in health care facilities to increase and strengthen PV activity such as the quality and quantity of adverse events report.
This workshop was held fully online with 2 invited speakers, Prof. Dr. Zullies Ikawati of Universitas Gadjah Mada's Faculty of Pharmacy and dr. Ahmad Hidayat, Health Management Consultant. As many as 85 participants from BPOM Officers, UPT BPOM, pharmaceutical companies, and healthcare professionals joined in this workshop. Mr. Yoshihiko Sano, Chief Officer of JICA Project told that Indonesian FDA, healthcare institutions and pharmaceutical companies should have awareness to check the real data in the real clinical settings. If they successfully developed drug safety including vaccines by way of PV, their safety would be absolutely increased and more secured. Director of Safety, Quality, end Export Import Control of Drug, Narcotic, Psychotropic, Precursor, and Addictive Substance, Dra. Tri Asti Isnariani stated that she expected for participants to improve their knowledge about reports of adverse event and drug side effect, and would take part in the next series of PV workshops of intermediate and advanced levels. She also asked all participants to collaborate in monitoring drug safety in Indonesia through the PV implementation in order to deliver benefits and contributions to patient safety as a form of public health protection in Indonesia.
Lecture session was started by PV definition and historical origin of PV from thalidomide tragedy explained by dr. Ahmad Hidayat. Based on his explanation, the lesson learnt from the tragedy was that several risks were not recognized when the drug product was approved for market due to the limited scientific evidences. As a result, several impacts are emerged following clinical practice. He emphasized the importance of conducting post-market monitoring activities to ensure that such situations should not happen again. Prof. Dr. Zullies Ikawati showed how to classify expected and unexpected adverse events reports based on healthcare professionals, pharmaceutical companies or individual evaluation. Then the afternoon session continued with regulations related to reports of adverse events and adverse drug reactions. The last session explained their procedures.
At the end of workshop, the organizer assisted each participant's group discussion with simulating reports of adverse events and adverse drug reactions using the e-MESO website. During the discussion session, some participants from pharmaceutical companies and healthcare professionals mentioned their concerns about the difficulties of reporting adverse events and implementing the procedures requested by e-MESO website. As a result, it was hoped that the module would be utilized as PV learning materials to deepen their understanding in reports of adverse events and adverse drug reactions.
 Prof. Dr. Zullies Ikawati, Apt, Faculty of Pharmacy Universitas Gadjah Mada
Prof. Dr. Zullies Ikawati, Apt, Faculty of Pharmacy Universitas Gadjah Mada
 Dra. Mayagustina Andarini, Apt, M.Sc., Head of Deputy for, Narcotics, Psychotropic, Precursors, and Addictive Substances Control of Badan POM
Dra. Mayagustina Andarini, Apt, M.Sc., Head of Deputy for, Narcotics, Psychotropic, Precursors, and Addictive Substances Control of Badan POM
 dr. Ahmad Hidayat, Grad, Dipl. Safety Sci., M.Sc.PH., Health Management Consultant
dr. Ahmad Hidayat, Grad, Dipl. Safety Sci., M.Sc.PH., Health Management Consultant
 Dra. Tri Asti Isnariani, Apt, M.Pharm, Plt. Director of Safety, Quality, end Export Import Control of Drug, Narcotic, Psychotropic, Precursor, and Addictive Substance
Dra. Tri Asti Isnariani, Apt, M.Pharm, Plt. Director of Safety, Quality, end Export Import Control of Drug, Narcotic, Psychotropic, Precursor, and Addictive Substance
 Mr. Yoshihiko Sano
Mr. Yoshihiko Sano
Chief Officer of JICA Project
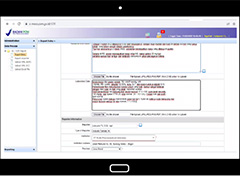 Data entry process in e-MESO website for the simulation session on adverse event
Data entry process in e-MESO website for the simulation session on adverse event
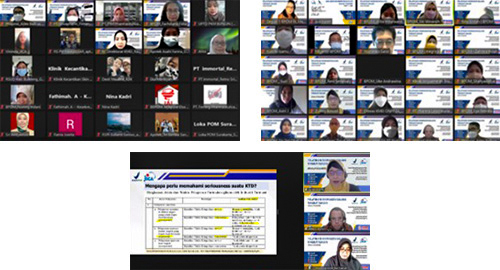 Lecture session by Prof. Zullies and dr. Ahmad
Lecture session by Prof. Zullies and dr. Ahmad
- About JICA
- News & Features
- Countries & Regions
- Our Work
- Thematic Issues
- Types of Assistance
- Partnerships with Other Development Partners
- Climate Change / Environmental and Social Considerations
- Evaluations
- Compliance and Anti-corruption
- Science and Technology Cooperation on Global Issues
- Research
- JICA Development Studies Program / JICA Chair
- Support for the Acceptance of Foreign HRs / Multicultural and Inclusive Community
- Publications
- Investor Relations
