Project News
2022-03-31
Completion of the Project
JICA-BPOM Project is going to be completed in the end of March 2022. The unprecedented pandemic caused difficulties for JICA operations in most countries, including Indonesia. Trainees travel to Japan was restricted, as was the travel of experts and consultants from Japan, based on the decision of the Japanese Government. It forced the cancellation of all training programmes under JICA's Knowledge Co-Creation Programme (KCCP) programmes operating in Japan.
Instead, JICA encouraged the provision of alternative programmes through other means, such as online platforms, cooperation with local experts and consultancy services. Since JICA collaborate with public institutions and universities in Indonesia, specialist capacity and research facilities have been developed and properly managed, and therefore the Project was able to provide such the alternative programmes to Badan POM in line with this principle.
During the pandemic, all online/offline alternative programmes provided by the Project drew on resources, including those affected by JICA inputs and those directly/indirectly affected by the inputs of Japanese experts. This is in line with JICA's goal of ensuring that locally available expertise, human resources, and research institutions are utilized to implement sustainable self-directed programmes in the field.
If it was in the normal situation, the Badan POM would not have sat in the mainstream on infectious disease control. In this pandemic the laboratories of Badan POM and Balai Besar POM were forced to be engaged in testing for COVID-19 national control programmes, and thus the Project supported the laboratory by providing equipment and several technical input for enhancing laboratory capacity and function. This Project extended it's original scope, contributed to infections disease control in Indonesia. And ultimate increase of laboratory capacity for testing drug and food using PCR systems and related advanced analytical equipment in Semarang, Bandung, Banda Aceh, Gorontalo, Denpasar, Surabaya, Mamuju, Makassar, Manado, Mataram, Lampung, Medan, Jambi, Ambon, Jayapura, Tarakan and PPPOMN laboratories.
The Project also focuses on small and medium-sized enterprises (SMEs). Supporting SME and enhancing food safety supervision is one of the central roles of the Indonesian FDA and produced materials for education and awareness will benefit to increasing technical knowledge and skills of stakeholders. Raising awareness of food safety in village community by producing a documentary film to encourage other villagers in Indonesia to gain knowledge they need and take similar initiatives on food safety in their own communities. Furthermore, enhancing capacity of inspectors for supervising SMEs was addressed in the Project by providing an interactive e-learning materials as a new internet-based learning tools to support Junior District Food Inspectors in all over Indonesia.
In drug safety part, our Project consistently conducted online PV workshop during this COVID-19 outbreak. Those workshops aim to increase understanding, competence and awareness of pharmacovigilance (PV) in pharmaceutical industry, healthcare professionals, and BB/BPOM (Badan POM Provincial Offices) to implement PV activity. PV inspection workshop for regulators was recently held by online, which contributes to improvement of Indonesian FDA officer capabilities and competency in terms of PV inspection through discussion and information sharing on Japan's cases. Last activity in this area was PV Basic Module workshop. In PV workshops 2020, several requests are raised from the participants to prepare level-graded PV learning materials to deepen their understanding. The basic level modules are expected to guide pharmaceutical companies, BPOM officers, especially healthcare professionals in health care facilities to increase and strengthen PV activity such as the quality and quantity of adverse drug reactions report. Based on this, JICA Project and BPOM conducted PV Basic Module Workshop as the first series of PV Graded Modules, with the aim of improving PV competence in these stakeholders to strengthen drug safety.
For more details about our activities, please visit our project news site.
Our team
Using an online platform, we worked always together in hybrid working arrangement over the pandemic period.
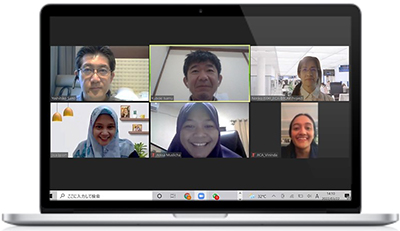 (Upper row, left to right) Mr Yoshihiko Sano, Chief Officer, Mr Isamu Kuboki, Administrative Officer, Ms Noriko Iseki Food Safety Expert, and (lower row, left to right) Ms Desny Putri Sunjaya, Secretary, Ms Anisa Muslicha, Project Officer, Ms Vininda Gelissa Buwono, English Writer.
(Upper row, left to right) Mr Yoshihiko Sano, Chief Officer, Mr Isamu Kuboki, Administrative Officer, Ms Noriko Iseki Food Safety Expert, and (lower row, left to right) Ms Desny Putri Sunjaya, Secretary, Ms Anisa Muslicha, Project Officer, Ms Vininda Gelissa Buwono, English Writer.




With some of our counterparts from Bureau of Cooperation Public Relations, Directorate for Community and Processed Food Enterprises Empowerment (PMPUPO), Directorate for Safety, Quality, and Export Import Control of Drug, Narcotics, Psychotropics, Precursors, and Addictive Substances (Ditwas. KMEI ONPPZA), National Quality Control Laboratory of Drug and Food (PPPOMN)
*(left to right)
- About JICA
- News & Features
- Countries & Regions
- Our Work
- Thematic Issues
- Types of Assistance
- Partnerships with Other Development Partners
- Climate Change / Environmental and Social Considerations
- Evaluations
- Compliance and Anti-corruption
- Science and Technology Cooperation on Global Issues
- Research
- JICA Development Studies Program / JICA Chair
- Support for the Acceptance of Foreign HRs / Multicultural and Inclusive Community
- Publications
- Investor Relations
