- Home
- Technical Cooperation Projects
- Index of Countries
- Asia
- Thailand
- The Partnership Project for Global Health and Universal Health Coverage Phase 2
- Project News
- Learning drug pricing: 2nd Fee Schedule Workshop
Project News
2021-09-14
Learning drug pricing: 2nd Fee Schedule Workshop
The project organized the 2nd workshop on fee schedule inviting two Japanese experts on 14 September 2021 online. Around 40 participants mainly from the National Health Security Office (NHSO), Food and Drug Administration of the Ministry of Public Health, and the Fee Schedule Committee, joined to learn drug pricing scheme under the Japan's fee schedule system and engaged in an active discussion.
One of the key challenges in Thailand raised at the 1st fee schedule workshop was drug pricing. Drug prices are one of the most important factors in Universal Health Coverage (UHC). The drug price determines whether or not it is included in the benefit package of a national health insurance, which in turn determines access to medicines. The drug price thus determines whether a particular disease will be treatable or not. Difficulty is to find the price of agreement among stakeholders; pharmaceutical companies must make a profit from the sales and invest in the development of new drugs, and healthcare providers including pharmacies need to get reimbursement from insurers or fund managers like NHSO at above-purchase price, while the government has limited financial resources.
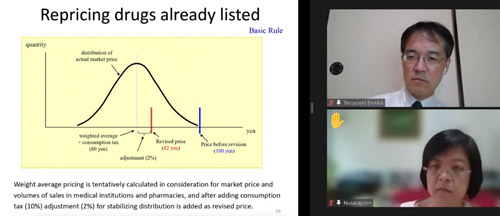
Following a presentation on Thai drug pricing policy and system, the Japanese expert showed the fundamental concept and the Japanese scheme of drug pricing to realize good quality, reasonable price, and adequate supply of medicines. The Participants attentively listened to the lecture and asked many questions on how to set and revise reimbursement price of listed drugs. They were particularly interested in the differences in pricing mechanism, for example, technical fees such as pharmacy dispensing fee and management fee are paid separately in Japan, whereas they are included in drug prices in Thailand. Topics such as how to set drug prices based on market price survey when revising the fee schedule, how to set prices of therapeutic drugs for rare diseases, etc. also gained particular attention.
Although the context of drug pricing in the two countries is different from one another - Japan has many drug discovery companies while Thailand mainly uses generic drugs -, we found that the two countries are facing some similar challenges. The project will arrange further opportunities to learn from each other on specific issues in depth to improve each system.
- About JICA
- News & Features
- Countries & Regions
- Our Work
- Thematic Issues
- Types of Assistance
- Partnerships with Other Development Partners
- Climate Change / Environmental and Social Considerations
- Evaluations
- Compliance and Anti-corruption
- Science and Technology Cooperation on Global Issues
- Research
- JICA Development Studies Program / JICA Chair
- Support for the Acceptance of Foreign HRs / Multicultural and Inclusive Community
- Publications
- Investor Relations
